
Source: link
Abstract
Microbial biomineralization is a key process in natural and anthropogenic environments. Certain bacteria and archaea produce cellular energy via anaerobic respiration using metals and metalloids as terminal electron acceptors, producing intra- and extracellular biominerals. This article explores the biomineralization of arsenic (As), iron (Fe), sulfur (S) and selenium (Se), in relation with microbial respiratory processes. Ferric iron (FeIII) and the oxyanions of As, S and Se are used as terminal electron acceptors by specialized bacteria and archaea, providing significant amounts of energy under anoxic and nutrient-limiting conditions. These transformations result in the formation of various types of arsenic sulfides, iron (oxyhydr)oxides and sulfides, elemental S/S0 and elemental Se/Se0 biominerals, which will be the focus of this review. Certain biominerals (e.g. S0) function as storage compounds; others, like Se0, may increase the density and the buoyancy of bacteria harboring them or are by-products of this process. Arsenic sulfides and iron (oxyhydr)oxides and sulfides appear to be by-product biominerals or have a yet unknown function. The use of these biominerals as biosignatures is an open topic and an ongoing debate. Further exploration of the reviewed biominerals is needed from both fundamental and applied viewpoints, aspects which will be covered in this review.
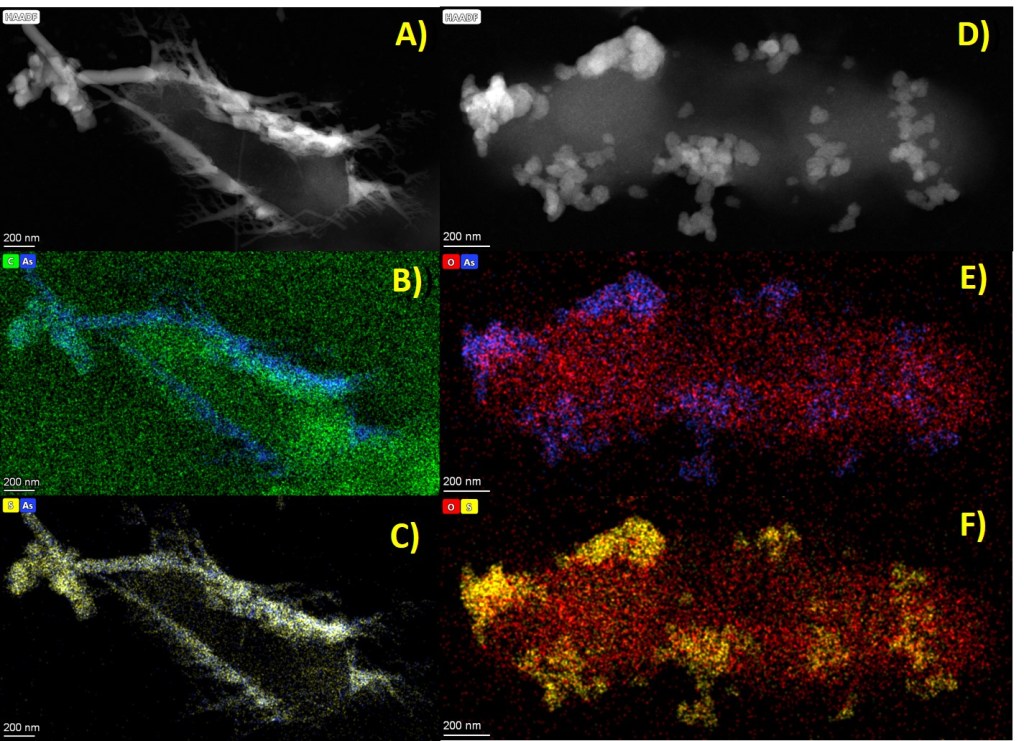
Biogenic AsS produced by Shewanella sp. O23S. Micrographs with the corresponding EDS elemental maps obtained from bacterial cells and biomineralization products: A) Scanning transmission electron microscopy (STEM mode) of nanorod (AsS) structures; B) Arsenic elemental mapping; C) Sulfur elemental mapping; D) Scanning transmission electron microscopy (STEM mode) of granular (As2S3) structures; E) Arsenic elemental mapping; F) Sulfur elemental mapping.

Summary of formation pathways for iron sulfides and the microorganisms involved. Fe(III)-reducers reduce various Fe(III) minerals to dissolved Fe2+. The reaction between dissolved Fe2+ and H2S released from sulfur-cycling microorganisms results in mackinawite precipitation. Its transformation to greigite and pyrite is accelerated in the presence of various intermediate sulfur species (S0, SSn2) produced by sulfur-cycling microorganisms and through the abiotic reaction between H2S and Fe(III) minerals.